Increasing the efficiency of mycoprotein fermentation

The fermentation process that creates mycoprotein products, like Quorn, can often be disrupted by the appearance of fungal variants which require the process to be halted. Dr Jordan Price, a senior specialist in fungal biotechnology at NIAB, explains how his research is helping to increase production capacity, as the demand for sustainable alternative protein products rise.
Understanding the fungal variants causing major disruptions in Quorn production.
Quorn is now one of the leading brands of alternative protein products. Quorn products are all made from mycoprotein which is protein made from the fungus Fusarium venenatum. Quorn gets its meat-like texture from the way that the fungus grows, which forms long, thin branches and is one of the main reasons for its popularity.
Mycoprotein production takes place in huge 150,000 litre fermentation towers, generating several tons of mycoprotein per hour. However, each fermentation process can only be run for a few weeks before highly branching variants of the fungus (known as C-variants) appear within the fermentation process. The highly branched nature of these C-variants negatively affects the texture of the final product, which ultimately limits production capacity.
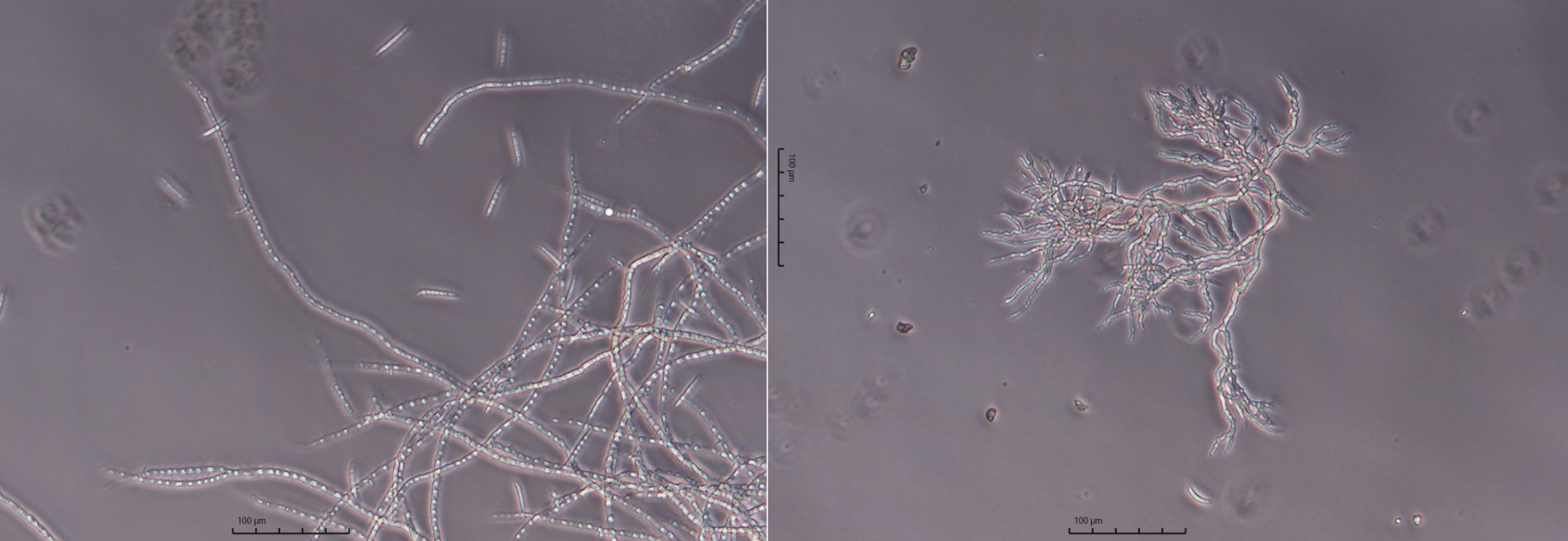
Left: F. venenatum wild-type culture under microscope. Right: highly-branched F. venenatum c-variant
This collaborative project between NIAB and Quorn producer Marlow Foods Ltd aims to understand and describe the branching patterns in F. venenatum to provide insight into the genetics that underpin the C-variant trait. This will help Marlow Foods to improve production efficiency, increase supply and ensure that Quorn remains one of the most sustainable meat alternatives on the market.
Mycoprotein, a low carbon meat alternative.
It has become increasingly clear that the continued increase in animal protein consumption is unsustainable. According to a report from the United Nations Food and Agricultural Organization (FAO), animal agriculture is responsible for:
- 14% of all human-associated greenhouse gas emissions.
- 40% of global methane emissions.
- 65% of nitrous oxide emissions.
Consumers can reduce their carbon footprint by switching from meat protein to alternatives such as mycoprotein, which releases up to 90% less greenhouse gases than beef production.
Understanding the mycoprotein c-variants that result in the fermentation process having to be stopped prematurely during production can help further decrease its carbon footprint. If we can delay or abolish the appearance of c-variants through developing new strains and innovating the production process, then each fermentation process could be extended. This in turn could make Quorn production far more efficient.
The results so far
We already have some very exciting preliminary results which suggest that the mutation of genes in a single biological pathway may be responsible for the C-variant trait. We’re currently using a gene editing approach to assess whether mutating one of these genes in the fungus is enough to cause the hyperbranching phenotype. There’s still some work that needs to be done to understand and confirm the role of this gene, but hopefully we will be writing this up in a scientific publication soon.
Further research to improve mycoprotein production
One of the most significant challenges to mycoprotein production is the reliance on a single feedstock upon which to grow the fungus. Currently, we have to use wheat-derived glucose, which requires special processing before it is suitable for use.
Previous work from the lab has revealed that the fungus can also grow on other feedstocks, for example beet-derived sucrose syrup, which can also enhance the production of several essential vitamins. However, in some cases, this leads to an increase in unwanted compounds.
If we can understand the relationship between different feedstocks and the fungus, then we could optimise fungal strains to be grown using different sources of feedstock. This would enable mycoprotein to be produced using a larger range of feedstocks, and could potentially enable the transition from an wheat-derived glucose feedstock to a UK-produced sucrose-based feedstock such as from sugar beet, and help to broaden the use of mycoprotein as an important component of global diets.

Left: Lab mycoprotein fermentation vessel. Right: Industrial mycoprotein fermentation vessels
Dr Jordan Price is a Fungal Biotechnologist at NIAB, based in Cambridge, part of the Growing Kent & Medway Alternative Protein Network: [email protected]
